Collection & Ordering
How it works
The Envisia classifier provides a more confident IPF diagnosis and ILD prognosis.
Collect patient samples

Ship to Veracyte
Veracyte provides sample collection kit and FedEx shipping from collection site to Veractye lab.
New simplified collection process!
Watch the collection video to hear about the easy process to collect a patient sample and order the Envisia test with a new on-line ordering process.
Who is a good candidate for Envisia?
Clinical Factors:
Have no known causes identified with suspected ILD
HRCT:
Probable UIP or indeterminate for UIP
Tolerability:
Able to tolerate standard TBBx procedure
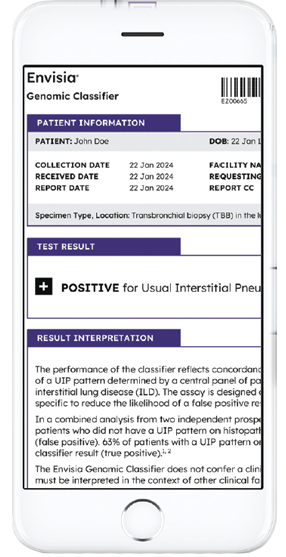
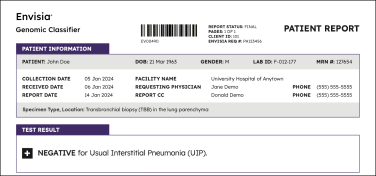
Result interpretation
Positive results
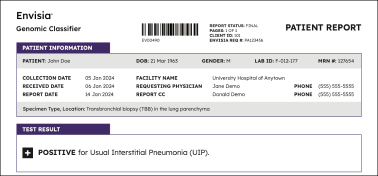
Negative results
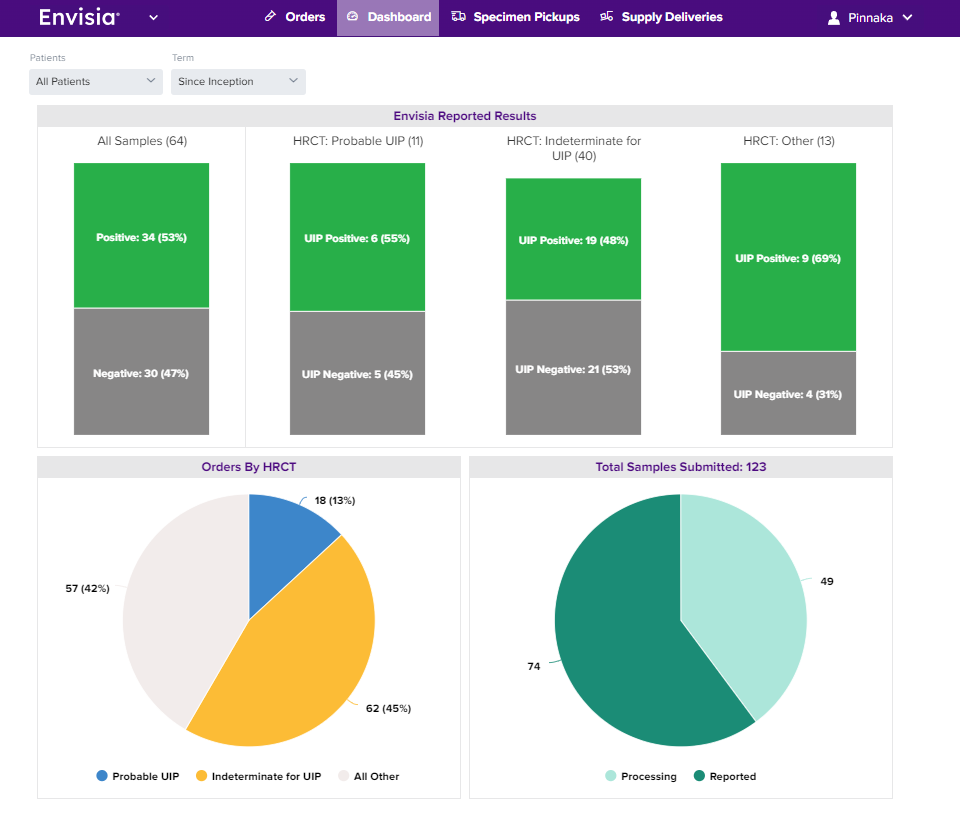
View your Veracyte portal to get practice insights, track trends, and better understand your patients.
Your Home Dashboard illustrates:
- Samples submitted and status report (Reported / Processing / Not Ordered)
- Order history
- Result breakdown by test result. Patient results are available within two weeks.
If you want to learn more or would like a walk through of your dashboard please contact our customer care team at 844.464.LUNG 844.464.5864 support@veracyte.com.
Covered by Medicare
The Envisia test is covered by Medicare. Veracyte bills Medicare and third-party payers directly.
The Veracyte Access Program provides financial support for both eligible uninsured and commercially available insured patients with financial need.
Frequently Asked Questions
Results for the Envisia Genomic Classifier are available within two weeks after the test order is confirmed.
Results can be accessed on the Veracyte online portal and mobile app.
Samples should be packaged and shipped to Veracyte on the same day as collected, using the provided shipper and return shipping labels.
Samples should be shipped the same day via FedEx®. Return label is pre-applied to the provided shipping box. If you do not have a routine pickup, call FedEx at 1.800.GOFEDEX (1.800.463.3339) to schedule one.
References
1. Raghu G, et al. Lancet Respiratory Medicine. Apr 2019.